Aging is often cited as the primary risk factor for a variety of diseases, both cognitive and physical. Scientists have thus come to recognize that if the aging process can be slowed down, many diseases – including Alzheimer’s – might be delayed or circumvented.
A March, 2022, conference in Copenhagen, “New Hallmarks of Aging,” addressed the nexus between aging and disease and came up with a new list of age-related conditions — or “hallmarks” — that once fully analyzed and researched may offer science more ways to slow the aging process and fight chronic cognitive diseases.
“Understanding the mechanisms of the aging process will therefore be pivotal to treat the root cause of multiple age-related diseases,” a paper summarizing the conference concluded.
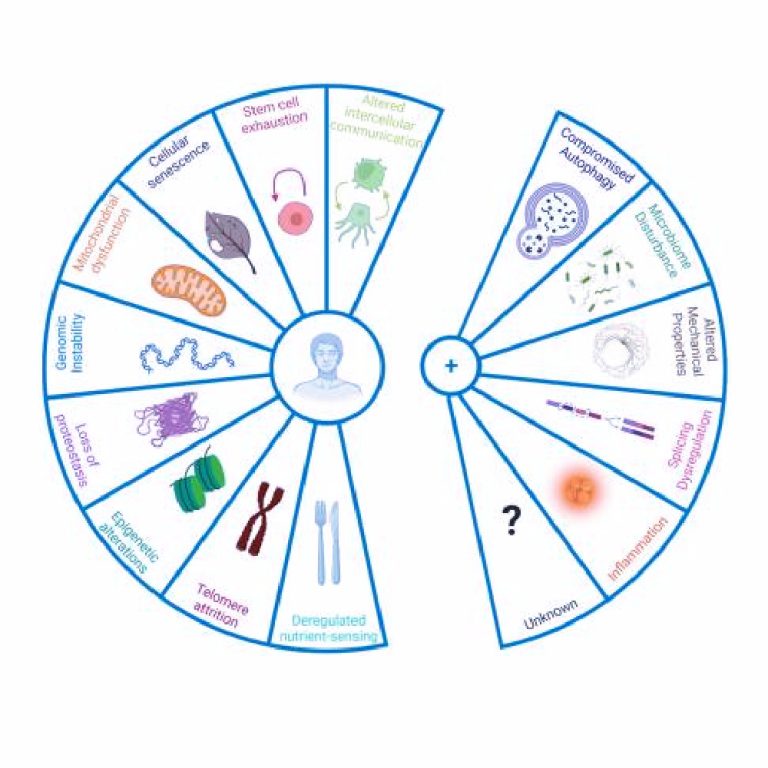
The five new hallmarks of aging have been added to nine existing hallmarks that were proposed in 2013 and have influenced and spearheaded research into the biology of aging. At the same time, they have been used to help integrate research on diseases of the brain as they relate to the aging process.
The five new hallmarks are:
— compromised autophagy (autophagy allows the orderly degradation and recycling of cellular components)
— microbiome disturbance (the disruption of microbiota in the body can lead to problems such as infection)
—altered mechanical properties (causing degradation of bodily parts and functions such as muscles, bones and connecting tissues)
— splicing dysregulation (involving metabolic and tumor pathologies, including breast cancer)
—inflammation (which is symptom of many chronic diseases, including arthritis and Alzheimer’s)
The Copenhagen conferees also reported that other emerging hallmarks are thought to exist that can be more specifically defined as research progresses.
The nine previously cited hallmarks are genomic instability, telomere attrition, epigenetic alterations, mitochondrial dysfunction, loss of proteostasis, deregulated nutrient-sensing, cellular senescence, stem cell exhaustion, and altered intercellular communication.
Researchers who see aging as the root of diseases like Alzheimer’s have usually focused on one approach or theory, or on a single hallmark at a time, even though the aging process is complex and involves intertwining pathologies.
“The aging process is multifaceted and multifactorial, and the panelists stressed that the classical approach of focussing on single pathways or even individual hallmarks might fail to capture the complexity and interconnectedness of aging,” the summary paper explained. Participants cited an urgent need to focus on the correlation between the hallmarks as research into aging continues.
The research also faces some very real obstacles. Conference participants warned, for instance, that some treatments, such as senolytics, may help prolong lifespans, but also may carry side effects that cause significant damage to organisms in the body.
Another subject discussed was that research into the aging process has not yet been fully developed. While acknowledging such a shortcoming, participants also cheered the new surge of research that has been initiated. That research and the introduction of the five new “hallmarks” will add another layer of scientific examination into Alzheimer’s disease and ways to slow the process of aging.